Study Overview
Rationale
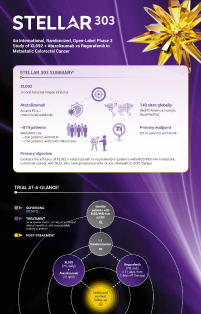
Microsatellite instability (MSI) is recognized as one of the major carcinogenetic pathways of colorectal cancer (CRC).1 Patients with MSI-high (MSI-H) CRC often respond to immune checkpoint inhibitors (ICI).2 However, only 4–5% of patients with metastatic CRC (mCRC) are MSI-H; most patients with mCRC are microsatellite stable (MSS) and typically do not respond to immunotherapy.2-4 Early phase studies of ICIs in combination with tyrosine kinase inhibitors have demonstrated encouraging efficacy and manageable toxicity in patients with previously treated MSS CRC,5,6 and emerging data suggest that patients without liver metastases are likely to derive the greatest benefit.7-10 This trial will assess the safety and efficacy of XL092 with atezolizumab or regorafenib monotherapy in patients with chemo-refractory mCRC who are MSS/MSI-low (MSI-L). The primary analysis will be overall survival (OS) in patients with non-liver metastases (NLM).
Design
Approximately 874 eligible patients from 140 sites globally (North America, Europe, and Asia Pacific) will be enrolled in STELLAR-303. Patients with MSS/MSI-L mCRC who have progressed during or after, or are intolerant to, standard-of-care (SOC) therapy will be randomly assigned to XL092 in combination with atezolizumab or regorafenib monotherapy to evaluate the effect of combination therapy on overall survival in patients with NLM. Eligible patients will be randomly assigned in a 1:1 ratio to each treatment arm. Approximately 350 patients with NLM will be enrolled, while enrollment of patients with liver metastases will be capped at ~524.