Study Overview
Rationale
Non–clear cell renal cell carcinoma (nccRCC) encompasses 25% of all RCC tumors. The most common histological subtype is papillary (15% of all RCC). Minor subtypes include translocation-associated RCC (≤1%) and unclassified (4%).1 For patients with nccRCC, there have been no dedicated, randomized phase 3 studies to adequately characterize benefit, thus a standard-of-care (SOC) therapy has not yet been established.
Design
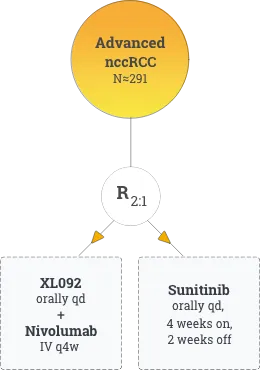
Approximately 291 eligible patients from 170 sites globally (North America, South America, Europe, and Asia Pacific) will be enrolled in STELLAR-304. Patients with unresectable, advanced, or metastatic nccRCC will be randomly assigned in a 2:1 ratio to XL092 in combination with nivolumab or to sunitinib to evaluate the effect of the combination therapy on progression-free survival (PFS) and objective response rate (ORR) versus sunitinib.
Stratification Factors
-
Presence of sarcomatoid features
-
IMDC prognostic score (favorable vs intermediate vs poor)